VYNDAMAX helps people with transthyretin cardiac amyloidosis (ATTR-CM) live longer
- VYNDAMAX* was studied in the “Tafamidis in Transthyretin Amyloidosis Cardiomyopathy Clinical Trial” (known as ATTR-ACT)
- The study tested if VYNDAMAX could help people with ATTR-CM live longer and reduce hospitalizations due to heart problems compared to placebo
- The study also tested the impact of VYNDAMAX on quality of life and ability to function
*The clinical studies of VYNDAQEL® (a different form of tafamidis) supported the approval of VYNDAMAX. Both drugs contain the same active ingredient.
How was VYNDAMAX studied?
The safety and efficacy of VYNDAMAX were studied in a 30-month clinical study of 441 adult patients with wild-type or hereditary ATTR-CM.
- The VYNDAMAX study primarily focused on the reduction of death and heart-related hospitalizations, as well as safety
- VYNDAMAX was also assessed on other aspects of ATTR-CM, including walking distance (6-Minute Walk Test) and health-related quality of life
- Efficacy and safety were determined by comparing patients treated with VYNDAMAX (n=264), with patients who did not take VYNDAMAX (placebo) (n=177)
- Patients were evaluated every 6 months for a total of 30 months
Survival rate at month 30
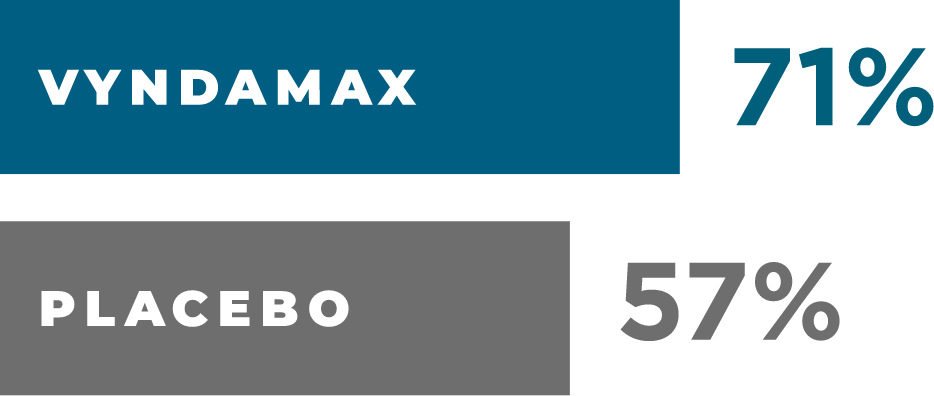
AFTER 21/2YEARS
more people were alive taking VYNDAMAX than placebo
VYNDAMAX was proven to reduce hospitalizations for heart problems
- In the study, patients taking VYNDAMAX had fewer heart-related hospitalizations than those in the placebo group
32% LOWER RISK
of hospitalizations related to heart problems

Better quality of life
(mental health status, enjoyment of life)
Fewer heart-related symptoms
(shortness of breath, fatigue, swollen feet or ankles)
More comfort performing daily activities
(ability to perform social and physical activities)
†At the end of the 30-month study compared to people taking a placebo. The Kansas City Cardiomyopathy Questionnaire (KCCQ) was used to measure health-related quality of life. Patients in the VYNDAMAX and placebo groups had worse KCCQ overall summary scores at month 30 than at the start of the study.
 While there's no cure today, VYNDAMAX slows down ATTR-CM
While there's no cure today, VYNDAMAX slows down ATTR-CM
Remember that even if you don’t feel it working, always take VYNDAMAX as prescribed. It’s important to tell your doctor how you are feeling.
Heart function and exercise capacity were measured in patients taking VYNDAMAX
The 6-Minute Walk Test (6MWT) measured heart function and exercise capacity.
At 30 months, people who took VYNDAMAX were able to walk a significant 83 yards farther (almost the length of a football field) on average than people who did not take VYNDAMAX (placebo).‡
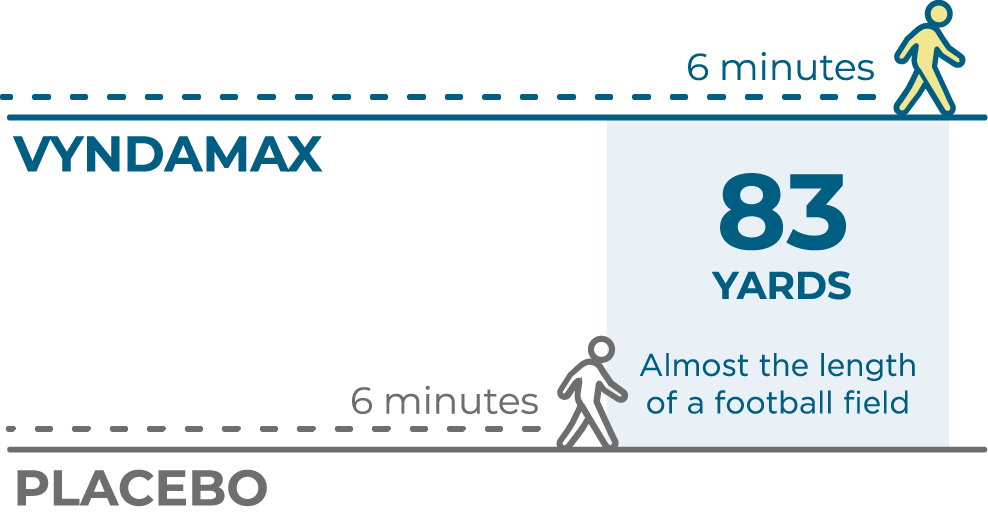 ‡Patients in the VYNDAMAX and placebo groups had worse 6-minute walking distance than at the start of the study.
‡Patients in the VYNDAMAX and placebo groups had worse 6-minute walking distance than at the start of the study.
Watch how VYNDAMAX can help you
Watch how VYNDAMAX can help you
Create a discussion guide to help you log your symptoms and talk to your doctor about ATTR-CM
Hear from Stan, a real patient taking VYNDAMAX
“I was glad to find out that there is treatment available now…so that gave me some hope.”
“I was glad to find out that there is treatment available now…so that gave me some hope.”
Get to the heart of what matters
Stay informed with educational materials about ATTR-CM and VYNDAMAX



